The process of metal corrosion is not something inevitable with fatal consequences. But it is imperative to fight it, to look for a way to remove rust. You can calmly watch how the nails on the fence have turned red, but if a water pipe has rusted, then it is better to quickly remove the rust before it ends in a leak and flooding of the neighbors on the floor below.

The content of the article:
- How and why rust forms
- What is the danger if you do not remove the rust?
-
Mechanical cleaning
- Sandblasting gun
- Aluminium foil
- Paint or dish scraper, metal sponge
- Abrasive materials
- Wire brushes, sponge brushes
-
Traditional methods
- Coca Cola
- Pork fat
- Fish fat
- Ketchup
- Baking and soda ash
- Caustic soda
- Baking soda
- Laundry soap and raw potatoes
- Hydrogen peroxide
- Kerosene
-
Chemical methods
- Sulfuric acid
- Hydrochloric acid
- Oxalic acid
- Acetic acid
- Wine acid
- Lactic acid
- Zinc chloride
-
Industrial production means
- Converters
- Electrolysis
- Solvents
- Heat treatment
- Results
How and why rust forms
The rusting process is the oxidation of iron, the transition from metal to oxides and hydroxides. For corrosion to form, 3 conditions are needed:
- pure metal - steel, cast iron without protective coating.
- water, always with dissolved salts.
- access of air (oxygen) to metal and water.
The rate of oxide formation increases several times if iron comes into contact with copper alloys. In this case, a pure galvanic couple is formed. Copper turns iron into rust at an accelerated rate. If a part (for example, a faucet) made of zinc or aluminum alloy comes into contact with an iron pipe or sink, or a steel bathtub, then the faucet will be destroyed first, not the pipe.
The rate and intensity of oxide formation directly depend on the composition of the water and the presence of impurities in the steel from which the pipe is made. There are not many salts in ordinary drinking water, and the exposed steel surface is passivated upon contact with air - covered with a protective microfilm. The process of oxide formation is inhibited.

But if the steel contains impurities of sulfur or phosphorus, then the rate of rust formation increases tenfold. The worse the quality of the metal, the faster corrosion occurs.
Another material can catastrophically speed up the process of rust formation. This is black vulcanized rubber, from which gaskets are usually cut for clamps on rusted heating pipes and steel water pipes. This is a temporary measure; the correct thing to do is to remove the clamp and rubber as soon as possible, otherwise the leak area will become covered with oxides.
What is the danger if you do not remove the rust?
The problem of rust is related to the deterioration of metal. If the causes are not removed correctly (at least not by blocking the access of air oxygen to steel or cast iron), then over time the iron will rust to holes. Moreover, the larger the rust layer, the better the conditions for the corrosion process.
Moreover, a layer of iron oxides collects and retains moisture, preventing it from evaporating. That is, a rusty spot on a pipe draws in water and condensate. Porous rust acts like a sponge; it adsorbs air, providing unhindered access of oxygen to the iron.
Therefore, if a small fistula or a rust spot appears on a steel pipe, this does not mean that the process is small and occurs only on the surface. Typically, corrosion continues to destroy metal even under a layer of paint or polymer sheathing.
When replacing water pipes, you can see that individual sections of them have been “eaten up” by rust so much that the wall thickness has decreased from 2 mm to 0.1 mm.
Mechanical cleaning
One of the easiest ways to properly remove rust from the surface of a steel part. The main disadvantage of mechanical cleaning is that along with the rust, you have to remove the top layer of metal, albeit a small one. But this forced measure is fully justified. The top layer of metal, as a rule, is already affected by microscopic pockets of rust, and it would be correct to remove it so as not to “treat” the steel, but simply paint it.
Sandblasting gun
One of the most effective ways to properly remove rust from a surface of any shape and size, even with protrusions, grooves, and curved profiles. Rust is removed by a strong air flow with washed and sifted sand.

Using a sandblasting gun, you can remove rust from surfaces of any shape and size. But the main advantage is that sand, like an abrasive, “eats away” even the smallest grains of oxides from microcracks, although only on the surface of the metal. At the same time, clouds of sandy dust rise in the air, so you need to work with safety glasses and a respirator.
A sandblasting gun is a container with a handle (with a valve) and a nozzle that sprays an air-sand mixture. The sand is poured inside the container, and a hose from an 8 bar air compressor is connected to the handle through a fitting.
You can make a gun with your own hands from a manual air gun, as in the video:
All that is required for the conversion is to remove the injector and correctly install a homemade atomizer made from the ceramic shank of an automobile spark plug onto the extension.
Aluminium foil
In order to remove rust, you will need foil for baking meat in the oven. You need to cut a thin strip, then wrap the section of pipe with rust in a thin paper napkin or a piece of toilet paper and moisten them with a weak vinegar solution.

Next, the foil strip is wound onto the paper sequentially, in one continuous layer. The ends are fixed with tape. The process of destruction of the foil and restoration of the metal will begin. Most likely, it will not be possible to completely remove rust, but you will definitely be able to block its growth.
Paint or dish scraper, metal sponge
Corrosion can also be properly removed by manual cleaning. For example, use a sponge made of metal mesh. Generally, the sponge is made from waste chromium alloy steel. Therefore, with its help you can remove quite a large amount of loose rust.
The lower layers, the hardest and densest, can be removed only by combining the action with a special reagent, such as an acid or a converter. This scheme ensures faster destruction of the rusted metal layer than if you simply rub it with a sponge or spray it with a liquid reagent.
Abrasive materials
Typically, abrasive materials refer to sandpaper. Emery, especially with large grains, is able to quickly and correctly remove even the hardest layers of rust. For preliminary stripping, it is better to use coarse paper with large grains, used for preliminary stripping of wood.
After the initial treatment of the rusty spot, it would be correct to use sandpaper with medium and fine grain. You can take an electric drill or a screwdriver and secure the paper to the attachment inserted into the chuck.

In this way, you can properly remove almost all corrosion and strip the rust-affected area down to white metal. But microtraces will still have to be removed using chemicals.
Wire brushes, sponge brushes
Many car enthusiasts believe that the correct way to remove rust is with wire brush attachments. They are used to quickly remove the old paint layer on bodies, clean soil and remove rust.
A round brush with twisted bronze or steel bristles is mounted on a shaft and installed in the chuck of an electric drill. Wire bristles remove large, loose rust faster and better than a sandblasting gun. But as soon as it gets to the lowest layer of corrosion, the removal efficiency decreases.
The brush, instead of cutting off the rusty layer, only polishes and compacts it. Therefore, it is correct to remove only the top layer of corrosion with a wire brush. You can try to remove the remaining rust using a wire sponge, but this will take 5-6 times longer than using chemicals.
Traditional methods
Most of the means and methods of combating corrosion from the people involve the use of products that are in every kitchen. Usually these are foods or substances with a pronounced acidic or alkaline base.
Coca Cola
The popular drink contains a fairly large amount of phosphoric acid and stabilizers that slow down the oxidation process of the product. Therefore, Coca-Cola can properly remove some of the rust, but it will require quite a large amount of the drink.

Pork fat
The product of the processing of interior lard is often used as a technical soap and tool care product. To do this, pork fat is boiled with a small amount of alkali or, more often, boiled with ash from burning sunflower stems. It turns out to be a very caustic soap. With its help, you can remove rust, wash away traces and at the same time preserve the instrument.
Fish fat
Most fish oil is an extract from fish bones and cartilage. It is extracted using a hydrocarbon solvent. Such material can be used as protection after corrosion has been removed mechanically or chemically. Fat penetrates deeply into microcracks, and in addition, passivates the metal. After treatment with fish oil, corrosion no longer appears.
Ketchup
There are quite a lot of varieties of ketchups and sauces: from sweet and sour to vinegar flavor. This means that the product contains a large amount of organic acids that can remove stains and convert iron oxides into water-soluble salts.

To remove rust, you need to choose the most liquid and acidic type of ketchup.
Of course, he will not be able to properly remove the rusty coating in 10 minutes. You will need to first remove most of the rust with a brush or steel sponge, mesh, remove dust and dip the part in ketchup. The cleaning period is at least 24 hours.
If you need to remove rust on earthenware, then it will be enough to apply the product to the rusty surface. Usually the marks start to go away after 40 minutes, but it's good to let it sit for a couple of hours.
Baking and soda ash
A mixture of two types of soda can properly remove rust hidden under a rather thick layer of organic plaque. The film blocks the access of chemicals to rust, so the plaque is removed mechanically.
The correct way to prepare a paste is to mix liquid soap and soda ash. All this is applied to the rust-affected area and cleaned with a brush with stiff bristles by hand.
In general, it would be correct to first remove the plaque with a paste, then rinse, remove the rust with an acidic agent, and only then repeat the treatment with the paste. Soda paste helps remove traces of acid, which itself is a strong catalyst for rust formation. Therefore, it is correct to use baking soda to neutralize the acid.
Caustic soda
This product, when in contact with water, gives a strong alkaline reaction, but in order for the iron oxides to react with soda, prolonged heating is required. Therefore, to properly remove rust, you need to heat a steel object and immerse it in a box of caustic soda. Once the part has cooled, it is transferred and immersed in hot water. After rinsing, the rust can be removed without leaving a trace.
Caustic soda can be used to clean earthenware toilets or any enamel-coated sink. For some reason, it is believed among experts that it would be more correct to make a paste from powder and water (but not hot), this makes it possible to remove rust both mechanically and chemically on rusty footprints.
Baking soda
This product is used exclusively for mechanical cleaning. You can often find descriptions of recipes for removing rust on earthenware using soda. This is not entirely correct, since soda does not react with iron oxides and hydroxides at room temperature. Rust can only be removed thanks to the mechanical abrasive effect of soda crystals.
Soda dissolves well in water. If you try to remove plaque with another abrasive, it will simply clog the drain. Removing the plug from the sump will be quite difficult.
Laundry soap and raw potatoes
There are such recipes. It is clear that it is almost impossible to remove rust from a metal surface using soap or raw potatoes. Moreover, it would not be entirely correct to even advise experimenting with these methods. Soap gives an alkaline reaction, potatoes are almost neutral. They will not be able to remove, much less dissolve, rust.
But soap, specifically laundry soap, and cut raw potatoes, when wetted and rubbed, form a viscous substance. Inorganic contaminants adhere well to it. So, it is correct to use them not to wash off iron oxides from the surface of a metal, but to remove rust stains from the surface of a sink or even from clothes.
After the procedure, you will need to additionally go through the cleaning area with a damp sponge or brush.
Hydrogen peroxide
The pharmaceutical preparation is used to treat wounds and cuts. It is impossible to remove rust with pure peroxide. Moreover, using a pure product to dissolve traces of corrosion is not entirely correct. When in contact with metal, peroxide releases very active oxygen, which will only add to the amount of rust.
To properly remove traces of corrosion, you need to use a mixture of peroxide and acid, and it is better to use organic and strong enough. For example, lemon.
You can properly remove rust using the following recipe:
- on the scale, 100 ml of hydrogen peroxide is poured into a plastic or glass container;
- here, in the container (controlled by weights), 20 g of citric acid is added;
- After keeping the solution for 1-2 minutes, parts with fairly strong rust can be immersed in the jar.
If everything is done correctly, the solution will heat up (up to 60-70OC), even boil from the gases released. Therefore, during processing, it would be correct to remove the jar somewhere on the balcony or take it out into the yard.
Most hydrogen peroxide is ordinary purified water, so lemongrass powder dissolves almost instantly. Peroxide significantly increases the chemical activity of the acid. It removes not only rust, but also paint. After completing the process, it is best to rinse the parts in clean water.
Kerosene
One of the supporting materials. Simply pouring kerosene on rusted metal is not entirely correct. It will not remove rust. But if you correctly apply kerosene in several approaches and leave it for 20-30 minutes, the rusty layer will slightly soften and swell. You can then remove it using a wire brush.

It is considered correct to use kerosene in cases where rust has “fused” with paint or, most often, with carbon deposits.
Chemical methods
The most stable and reliable effect is provided by acids and special preparations developed on their basis. But you also need to know how to use acids correctly.
Sulfuric acid
It cannot be purchased in a pure, highly concentrated form. The chemical is quite dangerous, and using it in its pure form to pickle rust stains is not entirely correct. You can purchase battery electrolyte. Its concentration will be enough to remove rust.

According to the technology, areas affected by corrosion must be cleaned of dirt and organic films, then dried and a small amount of acid applied with a brush to the metal surface.
The process goes quite quickly, the acid corrodes not only the oxides, but also the metal itself. Therefore, you need to correctly grasp the moment when to wash off the acid. This can be done with clean water, but it is correct to use an aqueous solution of baking soda.
Advice! You need to wash off the remaining sulfuric acid as thoroughly as possible, then wipe it with a dry cloth, then moistened with kerosene. Otherwise, after 10-15 minutes the metal will be covered with a thin layer of rust.
Hydrochloric acid
The chemical is also called “hodgepodge” and is used to clean old earthenware washbasins and washstands that are heavily soiled with rust stains. You can quite easily remove a layer of rust with pure acid (37%), but it would be more correct to prepare a 10% solution.
It is strictly forbidden to use “hodgepodge” for toilets and enamel washbasins. It will remove traces of rust, but at the same time the acid will remove the top layer of glaze, and the earthenware (or enamel) will begin to deteriorate.
Oxalic acid
The moderately strong acid is especially valued by restorers for its mild action, lack of side effects and relative safety of the product. The effect is similar to concentrated tomato juice, but a little stronger.
It is clear that you need to choose the right concentration of the reagent. To gently remove through rust on thin-walled objects, one spoon of acid per liter of clean water is enough. Increase processing time to 20 minutes.
You can often find recipes that give the proportion of 2 spoons per 200 ml of water. This is not entirely correct, the solution turns out to be too concentrated, and a lot of oxalates (salts) are formed, contaminating the metal surface.
Acetic acid
With a properly prepared solution, you can clean traces of rust on clothing. It is better not to clean with simple table vinegar; it can damage the fabric. A solution of the correct concentration is prepared at the rate of 4 liters of water and 50 ml of essence. After treatment, after about three hours, you need to wash the item completely, and not just the place where the stain was.

Vinegar essence can remove rust on metal surfaces. But this is not entirely correct, since the consumption of the chemical will be large, and the efficiency is not much higher than that of ordinary table vinegar.
In order to remove rust, you need to immerse the part in vinegar and leave it for at least 10 hours. After removing the vinegar and washing, traces of acetic acid (as well as salts) remain on the surface, so it would be correct to rinse with a soda solution, then with clean water.
Wine acid
Sold mainly in powder form under the name cream of tartar. Tartaric acid is somewhat weaker than citric or acetic acid. In addition, the drug often works as an inhibitor, that is, it slows down the action of other acidic agents.
Don't try to remove rusty deposits with cream of tartar. This is wrong, you can burn your fingers or skin.

The best option is a solution of 1 teaspoon of stone per glass of water. The part is left in the prepared solution for several hours, after which it must be thoroughly rinsed with clean water, even without soda.
Lactic acid
In its pure form it is practically unavailable. But you can use its natural analogue - liquid waste after squeezing fermented milk cheese or cottage cheese.
Lactic acid is relatively weak, in addition, there are many protein substances in such a liquid, they inhibit the reaction. Sometimes so much that the rust etching process stops.
Therefore, in order to properly remove corrosion products, the liquid must be preheated to 80OWITH. It will take at least 4-6 hours of hot treatment to remove rust.
Zinc chloride
It is used in the form of an aqueous solution. For preparation, a proportion of 50 g of zinc chloride per liter of water is used. A part with traces of rust is immersed in the solution for 2-3 hours. In order for the etching process to proceed without delay, the rust must first be cleaned off mechanically.
Industrial production means
If you need to remove a large amount of rust, then chemicals or folk remedies will not cope with the task, or too many etching materials will be required.
Therefore, when removing rust on car bodies, water pipes, and frame structures, it is correct to use special products.
Converters
There are quite a few ready-made rust removers. But almost all converters are prepared on the basis of an aqueous solution of orthophosphoric acid. It is a heavy, clear liquid, non-toxic, and if used correctly, is safe for humans.
Some converters have inhibitors added. But most often the manufacturing company adds regular washing powder to the converter. This is correct, since without it the converter does not wet out rust well.

To remove, you need to thoroughly moisten the layer of oxides and leave for a day. During this time, the chemical process will be completely completed. It is correct to keep the part under plastic film so that the water does not evaporate.
The next day, only a light film will remain in place of the treated rust. To properly complete the process, it must be well moistened with water. After three hours, a layer of iron phosphates forms in place of the rust. They are insoluble and protect the metal well from water and air oxygen.
Electrolysis
One of the most effective and safest ways to remove rust from small items made of steel or cast iron. To properly remove corrosion by electrochemical dissolution, you must adhere to the following rules:
- Use a powerful DC source, 1 cm2 the surface being treated must have at least 3-5 A of current load.
- To connect to a current source, you need to use wires with a large cross-section of cores, at least 25 mm2.
- A soda solution is used as an electrolyte, three teaspoons per 5 liters of purified water.
- The cathode (minus) is attached to a stainless steel electrode; its area must be at least 100 cm2. The anode is fixed on the part to be cleaned.
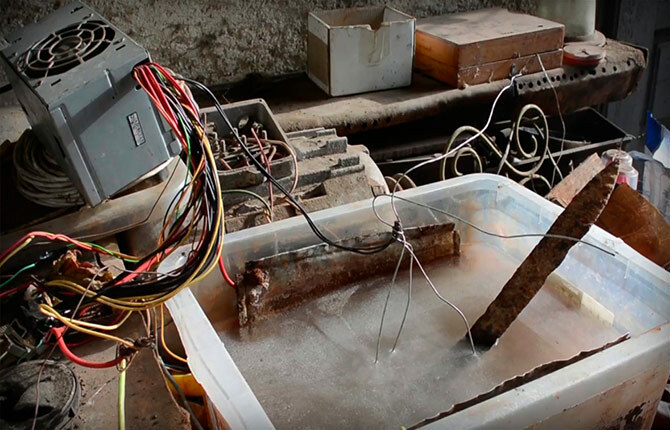
A distance of 5-6 cm is established between the anode and cathode; the electrodes must be firmly fixed on the walls of the container (5-6 liter plastic jar). An inverter or any 220/12 V transformer with a 200 A diode bridge can be used as a current source.
After turning on the transformer, the liquid in the container begins to boil intensely and heat up. A lot of carbon dioxide is released, so it would be correct to do the cleaning somewhere in a draft.
It takes 15-20 minutes to completely remove rust stains, but the oxides dissolve only on one side of the part, the one facing the opposite electrode. Therefore, in order to properly remove rust, the part will have to be turned 180O and repeat the cleaning process, but with fresh electrolyte.
Solvents
All existing brands of solvents give an almost neutral reaction, so it is almost impossible to remove a layer of rust from metal using them. But there are exceptions.
Rust on the surface of a product is not always formed due to corrosion of the walls of the part. All rusty marks on enamel cookware: teapots, saucepans, are formed as a result of the combustion and oxidation of iron carbonyls, which are always abundant in household gas.
In this case, traces of rust do not stick to the enamel, but to the varnish-fat layer on the outer walls. The same situation happens with enameled sinks and stainless steel sinks. Traces of foreign rust formed as a result of corrosion, for example, of water pipes, remain on the surface.
In this case, traces of corrosion can be properly removed with solvent P646 or P647. This treatment turns out to be more gentle than using cleaning pastes made from soda, borax and citric acid. In fact, P646 dissolves the varnish-fat base to which traces of oxidized iron have stuck. The enamel remains intact, without scratches on the gloss.
Heat treatment
Rust often appears on cast iron cauldrons, kebabs, and ducklings. For example, after another holiday in nature, a cauldron or cauldron made of cast iron is left in the open air, and accordingly, the walls inside very quickly become covered with rust.

You can properly remove rusty deposits by firing. All that is required is to remove the bulk of the plaque with a wire brush mounted on an angle grinder. Then rinse, dry to remove moisture and heat over low heat. To prevent the walls from oxidizing, when hot they are coated with a thin layer of linseed oil.
If you burn the oil correctly, the rusty coating will go away, and a non-stick coating of a burnished hue will form on the walls.
Results
There are many ways to properly remove rust. Not all of them are equally effective, but you can always choose the most suitable one for a particular case.
Tell us about your experience in removing rusty deposits. Which method do you think deserves more attention? Also share the article on social networks and bookmark it.


